Continuous Monitoring of Peripheral Edema for Heart Failure Management
Author: Deborah KesslerBodiGuide White Paper – August 2020
Abstract:
Heart failure is a major US health priority consuming more Medicare budget than any other diagnosis with 70% of those dollars being spent on hospitalization for emergency relief of dyspnea (breathlessness) associated with congestion of worsening heart failure. Heart failure management and treatment remain a difficult challenge, hampered by the absence of quantified methods for routine monitoring in the patient setting. A simple, reliable, quantitative tool for monitoring of peripheral edema in heart failure patients is needed.
Peripheral edema associated with fluid overload is present in approximately 70% of patients hospitalized for acute decompensated heart failure. Further, ankle circumference has shown to be an accurate and reliable measure of peripheral edema.
BodiGuide has designed, developed, and tested a prototype anklet and data management platform that meets the requirements necessary to provide accurate, reliable, and continuous monitoring of ankle swelling to patients, their caregivers, and clinicians. The device was worn by both healthy normal and heart failure subjects over many months to evaluate the feasibility of continuously wearing an anklet to collect and analyze ankle circumference measurements as a useful tool in the quantification and evaluation of peripheral edema and fluid status.
Fluid loading presents as deviations from a normal baseline or “dry state” and can be characterized to distinguish between compensated events and decompensation trends. These initial studies support the hypothesis that continuous monitoring of peripheral edema is feasible for monitoring fluid status to improve heart failure management.
Introduction
The Personal and Economic Burden of Heart Failure
Heart failure is a major US health priority currently estimated to afflict 6 million people and expected to impact 8 million by the year 2030 (Figure 1).1 Patients with heart failure experience a significant decline in quality of life and decreased mortality as the disease progresses. Fifty percent of people diagnosed with heart failure die within 5 years.2 Heart failure consumes more Medicare dollars than any other diagnosis and annual costs are estimated at $35 billion.3,4 Heart failure hospitalization accounts for 70% of all heart failure cost and is the leading cause of hospitalization over the age of 65.2,5–8
Acute Heart Failure Hospitalization
Acute Heart Failure (AHF) is defined as the progression or rapid onset of signs and symptoms of congestion or fluid overload. AHF may present as either a new onset (de novo HF) or worsening heart failure (WHF). Eighty-to-ninety percent of heart failure hospitalizations are a result of WHF.9 Dyspnea or breathlessness is the patient’s primary concern when seeking emergency care resulting in hospitalization (Figure 2)10.
Hospital Treatment
The treatment of patients in the hospital setting focuses on the use of diuretics to relieve symptoms while deferring diagnosis of the underlying pathophysiology to follow-up care.9. Relief of dyspnea and weight loss are poor indicators of decongestion and fluid overload is frequently still present at discharge11–17
Patient Impact
More than half of heart failure patients will be hospitalized three or more times within 5 years of diagnosis.3 As heart failure progresses, patients enter a cycle of crisis hospitalizations reflected in readmission rates of 22% within 30 days, 33% within 60 days, and 40% within 90 days.18 Each heart failure hospitalization indicates increased disease progression and is associated with an increased risk of death and reduced quality of life (Figure 3).11,19–21
A Better Method for Monitoring of Peripheral Edema is Needed
While diuretics are the cornerstone in managing fluid loading, a simple, reliable method for monitoring and evaluating diuretic response does not exist16. Like a blood pressure cuff for hypertension, a glucose meter for diabetes, or a thermometer for infection, a simple method for monitoring fluid status that can be performed in the patient setting is needed to improve patient self-care and provide the physiological feedback essential to prescribe and adjust therapies as the patient’s condition evolves. A quantitative measure of the patient’s fluid status would facilitate setting diuretic dosing to reduce excess interstitial fluid while balancing the risk to renal function.9,15,16,22 Patients need a simple method to monitor and recognize fluid retention that requires clinical intervention before it’s too late.23
Conventional Methods
Patients
Patients are currently instructed to monitor their daily weight or watch for ankle swelling24. While tracking daily weight appears to be a simple activity, adherence is low and baseline weights for comparison are not clearly established. And fluctuations in weight do not reliably represent changes in fluid volume.25 In the absence of a standard method to monitor ankle swelling, patients devise various subjective measures such as visibility of foot tendons, depth of sandal strap depression, or how tight their shoes fit.
Clinical Assessment
Right side cardiac catheterization is the preferred method of diagnosing congestion but the invasive nature of this technique limits its use in routine clinical practice.15,26 Clinicians use pitting edema or limb inspection to get a general assessment regarding the presence or absence of peripheral edema.27
Other approaches include data collected using cardiac implanted electronics devices such as CardioMems, defibrillators, and pacemakers.
Peripheral Edema
Peripheral edema is present in approximately 70% of patients hospitalized for acute decompensated heart failure (Figure 5).10,28 Further, ankle circumference has shown to be an accurate and reliable measure of peripheral edema.29 Accordingly, consideration for continuous monitoring of peripheral edema for WHF patients at risk for hospitalization and following discharge is warranted.
Closing the Loop
The ability to successfully monitor and interpret peripheral edema as a method to evaluate a patient’s fluid status will provide the missing link in the management of worsening heart failure. Heart failure patients would have a tool to assess their status and adjust their medication, similar to the way diabetics use blood glucose to adjust insulin. Continuous monitoring of peripheral edema would provide cardiologist with longitudinal data important to tracking and managing their patient’s disease progression. And early detection of decompensation would trigger the intervention necessary to break the cycle of crisis hospitalizations.
Monitoring of Peripheral Edema
A careful study of the factors involved in the measurement of ankle swelling establishes a specific set of criteria that must be met to achieve accurate and reliable measurements. This measurement data can be translated into meaningful information utilized by patients to motivate self-care or by clinicians to monitor and evaluate the effectiveness of prescribed therapies (Table 1).
Technical Requirements
For reliability and accuracy, the device must maintain optimal tension during the entire range of expansion and contraction independent of ankle size such that compression of the limb does not occur. Measurement accuracy must be sufficient to distinguish fluid loading events and trends from normal circumference variation that occurs when limb orientation changes from horizontal to vertical. The device must account for location of the circumference measurement on the limb.
Usability
For comfort and continuous use over long periods of time, the device must be passive, unobtrusive, and simple to place around the ankle. The device must also be waterproof and have sufficiently long battery life such that patients rarely need to remove the device for charging. The device must accommodate a wide range of ankle sizes and measure absolute circumference to achieve inter-operability.
Reliable Data and Information
The system must provide the right information to the right person at the right time whether this is the patient, the patient’s caregiver, chronic care management organizations, or the patient’s clinical team. Patients and their caregivers must be able to associate the impact of self-care activities such as medication, diet, and exercise on fluid status. The system must provide continuous measurement and monitoring of ankle swelling and recognize patterns that deviate from the patient’s normal patterns or baseline. When associated with prescribed medication, the fluid volume history must provide the cardiologist with the opportunity to determine therapeutic effectiveness and optimize treatment over the natural course of the disease.
Business of Healthcare
Equally important to the technical, usability, and effectiveness requirements, a device will only be successful if the healthcare system can afford to deploy the device to the broad populations that can benefit from it.
Ankle Swelling Monitoring Criteria
Technology
- Accuracy
- Tension without compression
- Limb Orientation
- Measurement Position
- Sampling Frequency
Usability
- Passive
- Comfortable
- Biocompatible
- Waterproof
- Battery Life
- Sizing
- Interoperability
Meaningful Data and Information
- Normal Baseline
- Deviations
- Trends
Business of Healthcare
- Affordable
- Economical
The BodiGuide Anklet
The team at BodiGuide has designed, developed, and tested a prototype anklet and data management platform that meets the requirements necessary to provide accurate and reliable continuous monitoring of ankle swelling to patients, their caregivers, and clinicians.
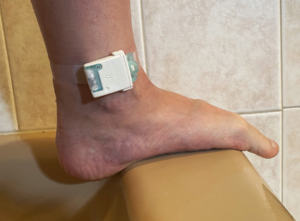
BodiGuide worked with both healthy normal and heart failure subjects over many months to evaluate the feasibility of continuously wearing an anklet to collect and analyze ankle circumference measurements as a useful tool in the quantification and evaluation of peripheral edema and fluid load.
System Design
The BodiGuide system consists of four components: an anklet, a data gateway, a software backend for storing and managing data, and signal processing algorithms to translate circumference readings to meaningful information. Circumference measurements are obtained using a proprietary position sensor able to detect small variations in expansion or contraction of the anklet with an accuracy of 1mm. Consistent tension is achieved without compressing the ankle using a constant, low-force spring. The anklet also includes an accelerometer for capturing limb orientation and patient activity.
Patient Comfort and Ease of Use
All test subjects report ease of use and quickly forget they are wearing the device. The anklet is waterproof and the battery last over 6 months. No action other than wearing the anklet is required on behalf of the subjects.
Signal Processing
Circumference readings are collected multiple times per hour, in addition to limb orientation, to generate a daily swelling profile which reflects the changes in ankle circumference that naturally occur when a person goes from a horizontal position during sleep to a vertical position throughout the day. A normal baseline is established for the patient during a startup period that is adjusted based on his/her state (dry or wet) at the time of installation. The system continuously monitors ankle swelling and recognizes patterns that deviate from the patient’s normal baseline.
Results
Initial studies support the hypothesis that continuous monitoring of peripheral edema is feasible for monitoring fluid status to improve heart failure management. Fluid loading presents as deviations from a normal baseline or “dry state” and can be characterized to distinguish between compensated events and decompensation trends (Figure 4).
Well-managed heart failure patients present a daily swelling profile and normal baseline consistent with healthy normal subjects. Figure 4a is an example of a normal baseline associated with a repetitive daily oscillating swelling profile that is typical. The anklet detects changes in interstitial fluid distribution in response to limb orientation and gravity as the subject’s limb changes from a horizontal position while sleeping to a vertical position throughout the day. Compensated fluid retention such as missing daily medication or isolated high-salt intake are observed as a shift in the daily swelling profile relative to the normal baseline and a return to normal after the subject corrects the anomalous behavior. Figure 4b illustrates the disruption that can occur when the subject ingests a high-salt meal, in this case a ham sandwich. Potential decompensated fluid retention is observed as an increasing trend consistent with fluid accumulation or overload and indicates a medical assessment and intervention may be needed (Figure 4c).
Continuous monitoring of anklet swelling combined with limb orientation captures the dynamics and magnitude of interstitial fluid volume. The precision of the BodiGuide anklet presents the opportunity to accurately detect variations and trends in fluid volume.
Conclusion
Heart failure patients follow a pattern of clinical stability punctuated by episodes of worsening symptoms that require hospitalization where treatment focuses on the relief of patient discomfort, rather than recognizing and addressing the underlying cause of the instability. The management and treatment of acute heart failure remains a difficult challenge hampered by the absence of quantified methods for routine monitoring in the patient setting. Unlike hypertension or diabetes, heart failure patients do not have an easy, reliable objective measure to report disease status to their physicians.
Reliable, accurate, continuous monitoring of fluid volume creates an opportunity to distinguish between swelling events related to short term variations that resolve on their own or can be managed with improved self-care and those associated with disease progression that require diagnosis and clinical intervention. The availability of reliable, patient-generated data provides the cardiologist with a tool to evaluate therapeutic effectiveness and optimize treatment over the natural course of the disease.
Critical to the success of this approach is the necessity for the monitoring device to be simple to use and require minimal-to-no effort on the part of the patient.
Bibliography
- Heidenreich, P. A. et al. Forecasting the Impact of Heart Failure in the United States: A Policy Statement From the American Heart Association. Circ. Heart Fail. 6, 606–619 (2013).
- Mozaffarian, D. et al. Heart Disease and Stroke Statistics—2016 Update. 323.
- Dunlay, S. M. et al. Lifetime Costs of Medical Care After Heart Failure Diagnosis. Circ. Cardiovasc. Qual. Outcomes 4, 68–75 (2011).
- Liang, L. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2017. 18.
- Berry, C., Murdoch, D. R. & McMurray, J. J. V. Economics of chronic heart failure. Eur. J. Heart Fail. 3, 283–291 (2001).
- Cohen, S. Statistical Brief #455: The Concentration of Health Care Expenditures and Related Expenses for Costly Medical Conditions, 2012. 8.
- Stewart, S. et al. The current cost of heart failure to the National Health Service in the UK. Eur. J. Heart Fail. 4, 361–371 (2002).
- Lesyuk, W., Kriza, C. & Kolominsky-Rabas, P. Cost-of-illness studies in heart failure: a systematic review 2004–2016. BMC Cardiovasc. Disord. 18, 74 (2018).
- Butler, J., Braunwald, E. & Gheorghiade, M. Recognizing Worsening Chronic Heart Failure as an Entity and an End Point in Clinical Trials. JAMA 312, 789–790 (2014).
- Goldberg, R. J. et al. Symptom Presentation in Patients Hospitalized with Acute Heart Failure. Clin. Cardiol. 33, E73–E80 (2010).
- Gheorghiade, M. et al. Pathophysiologic Targets in the Early Phase of Acute Heart Failure Syndromes. Am. J. Cardiol. 96, 11–17 (2005).
- Lala, A. et al. Relief and Recurrence of Congestion During and After Hospitalization for Acute Heart Failure: Insights from DOSE-AHF and CARRESS-HF. Circ. Heart Fail. 8, 741–748 (2015).
- O’Connor, C. M., Stough, W. G., Gallup, D. S., Hasselblad, V. & Gheorghiade, M. Demographics, Clinical Characteristics, and Outcomes of Patients Hospitalized for Decompensated Heart Failure: Observations From the IMPACT-HF Registry. J. Card. Fail. 11, 200–205 (2005).
- Vaduganathan, M. et al. Hemoconcentration-guided Diuresis in Heart Failure. Am. J. Med. 127, 1154–1159 (2014).
- Mullens, W. et al. The use of diuretics in heart failure with congestion — a position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 21, 137–155 (2019).
- Kristjánsdóttir, I., Thorvaldsen, T. & Lund, L. H. Congestion and Diuretic Resistance in Acute or Worsening Heart Failure. Card. Fail. Rev. 6, e25 (2020).
- Rubio-Gracia, J. et al. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int. J. Cardiol. 258, 185–191 (2018).
- Kilgore, M., Patel, H. K., Kielhorn, A., Maya, J. F. & Sharma, P. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag. Healthc. Policy 10, 63–70 (2017).
- Setoguchi, S., Stevenson, L. W. & Schneeweiss, S. Repeated hospitalizations predict mortality in the community population with heart failure. Am. Heart J. 154, 260–266 (2007).
- Mills, R. M. The Heart Failure Frequent Flyer: An Urban Legend. Clin. Cardiol. 32, 67–68 (2009).
- Nieminen, M. S. et al. The patient perspective: Quality of life in advanced heart failure with frequent hospitalisations. Int. J. Cardiol. 191, 256–264 (2015).
- Greene, S. J., Mentz, R. J. & Felker, G. M. Outpatient Worsening Heart Failure as a Target for Therapy: A Review. JAMA Cardiol. 3, 252 (2018).
- Riegel, B. et al. State of the Science. Circulation 120, 1141–1163 (2009).
- Managing Heart Failure Symptoms. www.heart.org https://www.heart.org/en/health-topics/heart-failure/warning-signs-of-heart-failure/managing-heart-failure-symptoms.
- Testani, J. M. et al. Substantial discrepancy between fluid and weight loss during acute decompensated heart failure treatment: Important lessons for research and clinical care. Am. J. Med. 128, 776-783.e4 (2015).
- Gheorghiade, M. et al. Assessing and grading congestion in acute heart failure: a scientific statement from the Acute Heart Failure Committee of the Heart Failure Association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur. J. Heart Fail. 12, 423–433 (2010).
- Trayes, K. P., Studdiford, J., Pickle, S. & Tully, A. S. Edema: Diagnosis and Management. Am. Fam. Physician 88, 102–110 (2013).
- Yancy, C. W., Lopatin, M., Stevenson, L. W., De Marco, T. & Fonarow, G. C. Clinical Presentation, Management, and In-Hospital Outcomes of Patients Admitted With Acute Decompensated Heart Failure With Preserved Systolic Function. J. Am. Coll. Cardiol. 47, 76–84 (2006).
- Brodovicz, K. G. et al. Reliability and Feasibility of Methods to Quantitatively Assess Peripheral Edema. Clin. Med. Res. 7, 21–31 (2009).